Many types of research require peptides from over 50 to up to 150 amino acids. The challenge in the synthesis of such long peptides is to overcome the enormous cumulative effect of the yield. Synthetic inefficiencies associated with peptide aggregation, such as slow or incomplete coupling and/or deprotection reactions, illustrate the complexity that often arises. A second issue in long peptide synthesis is poor solvation during assembly of the protected peptide.
For successful synthesis of long peptides it is essential to overcome the aforementioned problems, for which multiple of strategies have been described and are applied by Pepmic. These include high-swelling resins with low peptide loadings, pseudoproline building blocks, fragment condensation, native chemical ligation and others.
Pepmic scientists will thoroughly evaluate the target peptide sequence, and select the most promising synthetic approach. Over time, Pepmic has obtained a lot of experience in the successful synthesis of challenging long peptides, and developed these into well documented protocols. These ensure a high success rate, even for peptides with lengths of up to 100 amino acids or more.
For long peptides, Pepmic frequently makes use of fragment synthesis and chemical ligation technologies, by which the unprotected peptide chains react chemo-selectively in aqueous solution. The most common form of chemical ligation involves a peptide thioester that reacts with a terminal cysteine residue. This synthesis strategy is routinely implemented within our synthesis platform, and allows to successfully synthesize long peptides.
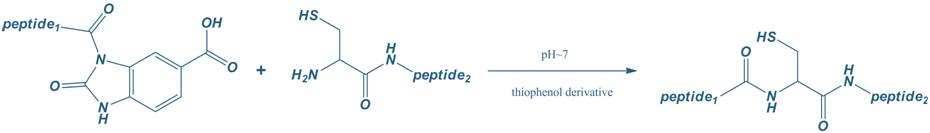 Fragment Synthesis and Chemical Ligation Technologies for Long Peptide Synthesis Fragment Synthesis and Chemical Ligation Technologies for Long Peptide Synthesis
|
