To improve peptide pharmacokinetics, conjugating the peptide to lipids is a favored approach. It prolongs the half-life in the circulation significantly. The most typical derivatization involves long-chain fatty acids. Fatty acid conjugated peptides can also be used for a number of different applications, e.g. for increasing their antibacterial activity or eukaryotic cell toxicity.
For fatty acid conjugation various approaches are available. Synthesis can be performed where fatty acids are either conjugated to the N-terminus, or to the side-chain of a lysine. Also the cysteine residues in peptides can be modified with fatty acids, giving the corresponding thioester derivatives. The fatty acids that are most commonly used are: Caprylic acid (C8), Capric acid (C10), Lauric acid (C12), Myristic acid (C14), Palmitic acid (C16) or Stearic acid (C18).
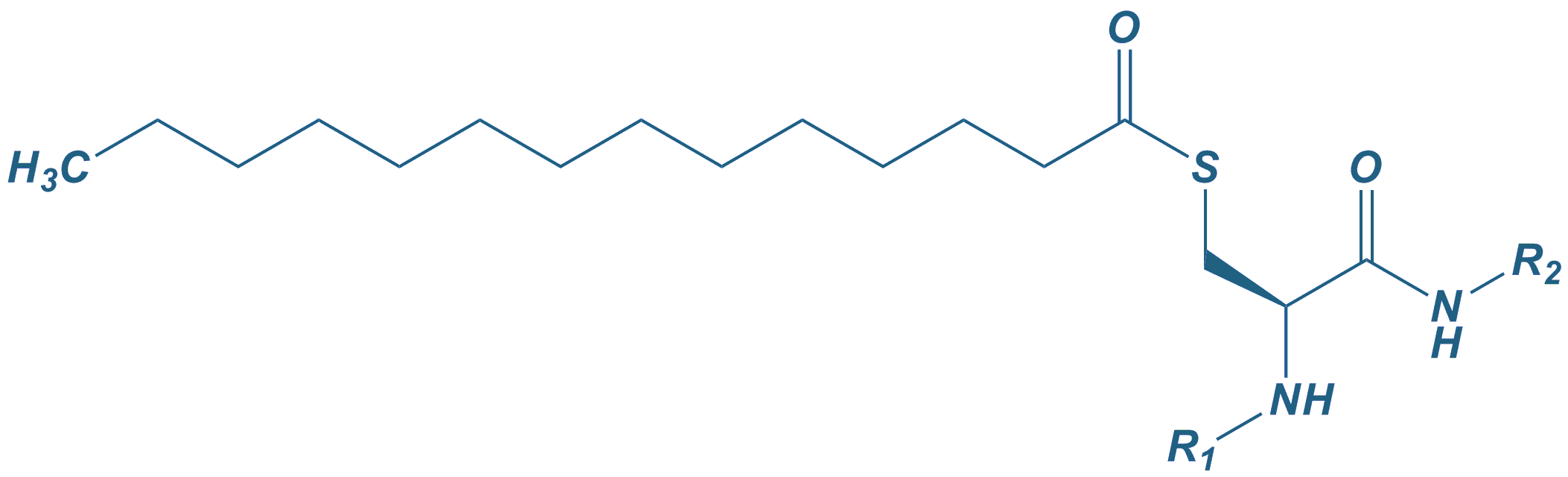
Myristoyl thioester
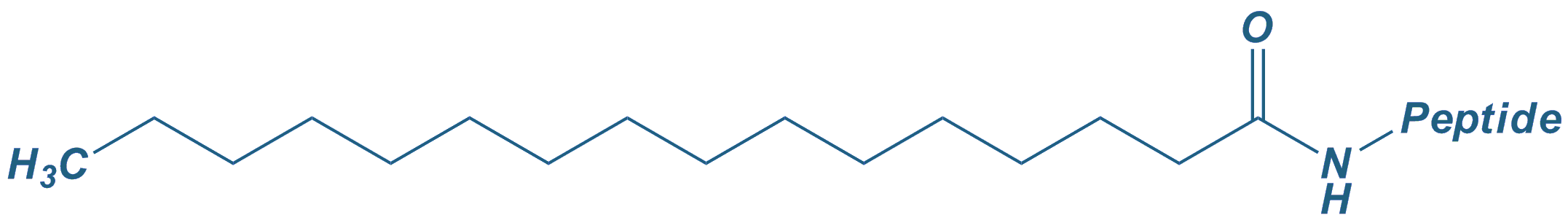
Palmitoyl ester
Cholesterol-conjugated peptides
Next to fatty acid conjugation, derivatization with cholestol is applied to improve peptide pharmacokinetics. Moreover it has been reported to increasing the potency of antiviral peptides through the introduction of a cholesterol group membrane anchor.
Cholesterol can be conjugated to a peptide via an N- or C-terminal inserted cysteine. For this, we use a cholesterol derivative that has been modified with a cysteine-reactive 2-bromoacetyl moiety.

Palmitoyl ester
|
