Apart from the 20 natural L-amino acids, there is a multitude of non-natural or unusual amino acids available that can be built into synthetic peptides. There are many different reasons to incorporate non-natural amino acids, such as for example to enhance affinity, selectivity of stability of peptide drug leads. Another application is the use of non-natural amino acids for induction or stabilization of secondary structures (α-helices, β-sheets, β-turns).
Pepmic offers a large number of different non-natural amino acids, which we routinely incorporate into peptides. These include D-amino acids, homo amino acids, beta-homo amino acids, N-methyl amino acids, alpha-methyl amino acids, non-natural side chain variant amino acids and other unusual amino acids.
D-amino acids involve the mirror image of the naturally occurring L-isomers. They are used for a range of applications, mostly to increase resistance against a range of degradation enzymes. Peptides containing D-amino acids are therefore significantly more stable than peptide containing only L-amino acids. In some cases peptides containing D-amino acids display increased biological activities as compared to their natural L-variant.
Homo-amino acids: The prefix “homo” to the name of an amino acid indicates the addition of a methylene (CH2) group to the α-carbon of an amino acid. These are used to creating peptides that may have altered biological characteristics, such as enhanced biological activity or better biological stability.
Beta-homo-amino acids are analogs of standard amino acids in which the carbon skeleton has been lengthened by insertion of one carbon atom immediately after the acid group. Incorporating beta-homo-amino acids into bioactive peptides can improve their pharmacological properties. Increase of in vivo half-life is a known effect of beta-homo-amino acid incorporation. Beta-homo-amino acids can increase potency, increase selectivity and reduce toxicity.
Unusual amino acids occur most frequently in microbial peptides and proteins and are formed posttranslationally. The unusually amino acids often contribute to the special bioactivity of these peptides. Pepmic is able to incorporate a wide range of unusual amino acids.
Prominent examples thereof are: citrulline (Cit), hydroxyproline (Hyp), norleucine (Nle), 3-nitrotyrosine, nitroarginine, ornithine (Orn), naphtylalanine (Nal), Abu, DAB, methionine sulfoxide or methionine sulfone. In drug development unusual amino acids are used to enhance the pharmaceutical properties of a peptide drug lead.
Name
|
Structure
|
Alpha-Methyl Phe
|
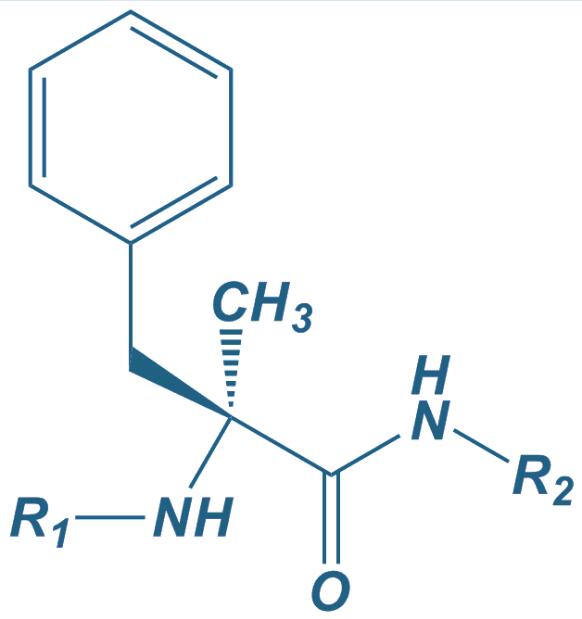
|
Beta-Homo-Phe
|
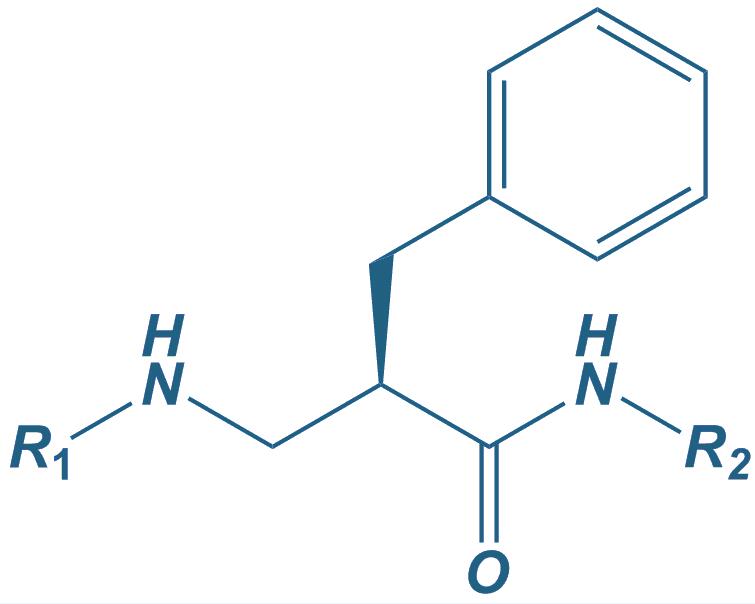
|
Citrulline
|
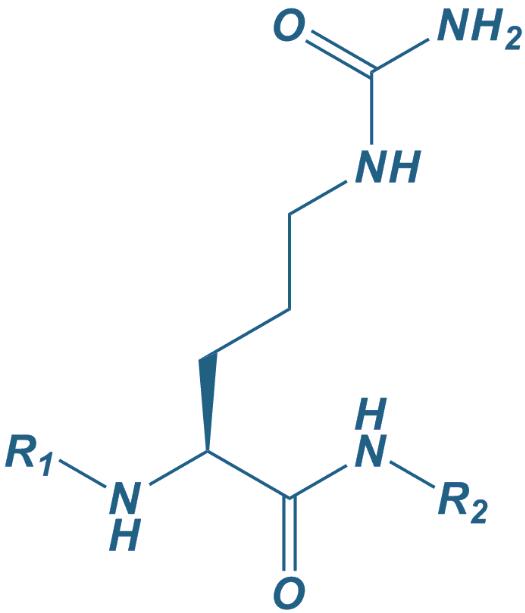
|
Cys(Acm)-OH
|
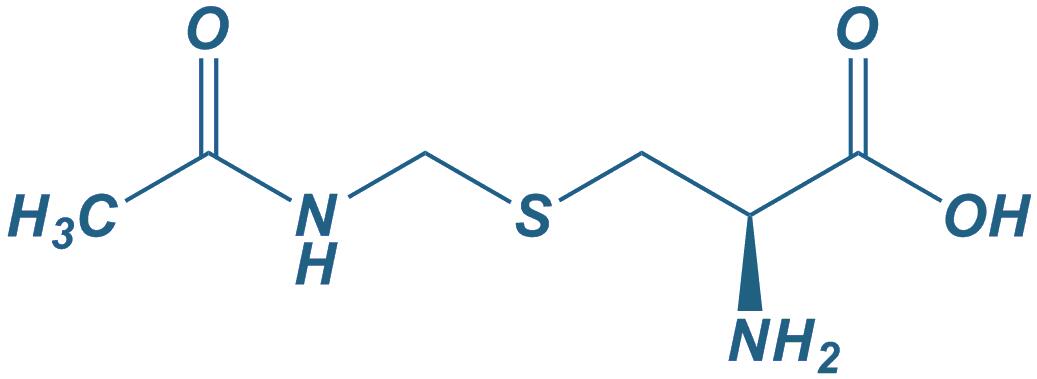
|
Cysteine(iodoacetamide)
|
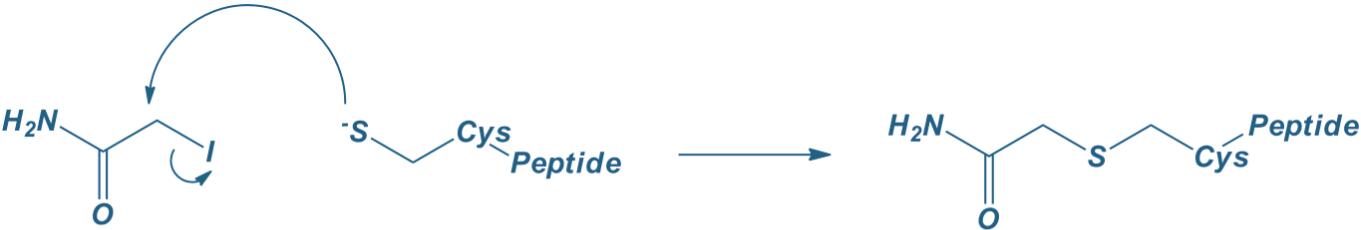
|
D-Phe
|
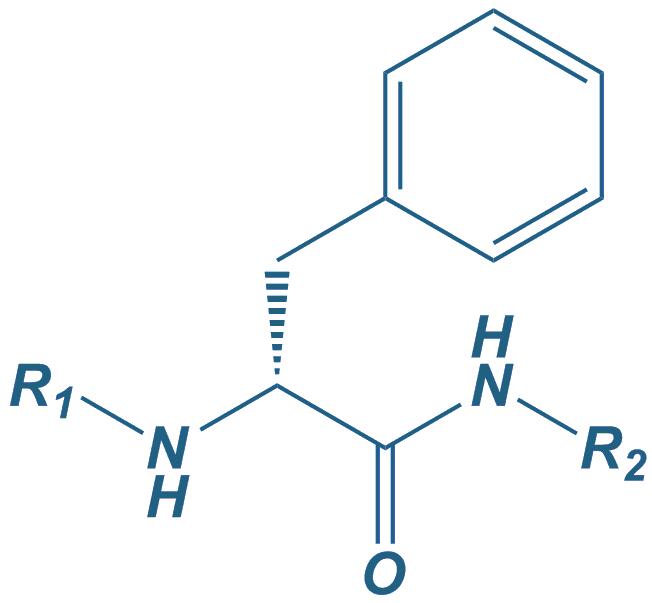
|
DOPA,3,4-Dihydroxyphenylalanine
|
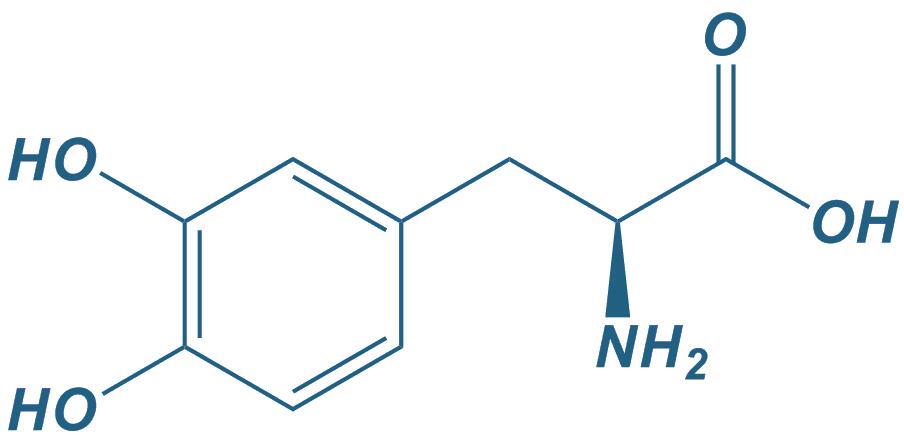
|
H-Lys(Z)-OH
|
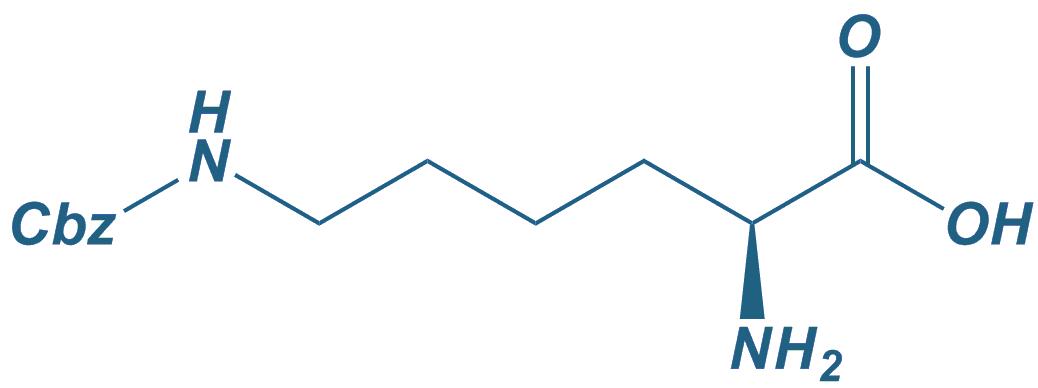
|
H-Lys(Z)-OMe
|
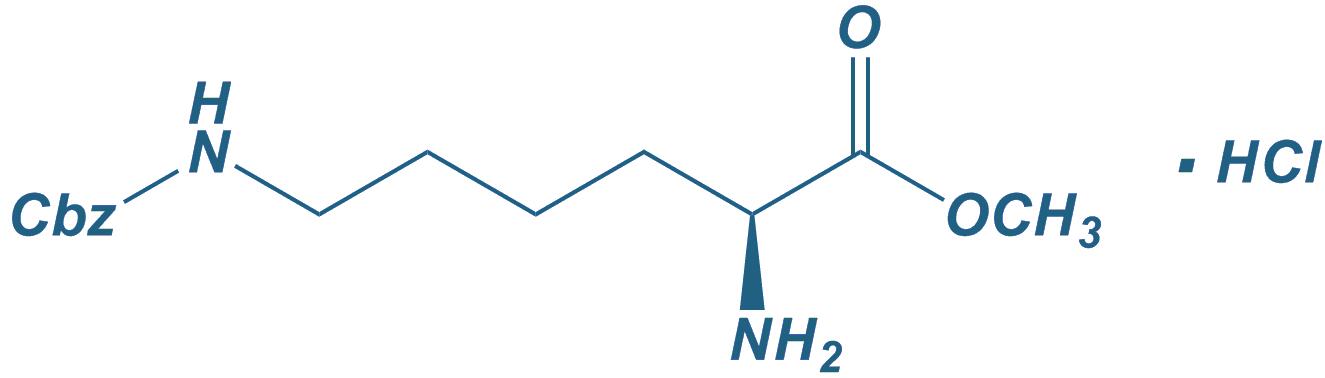
|
Homo-Phe
|
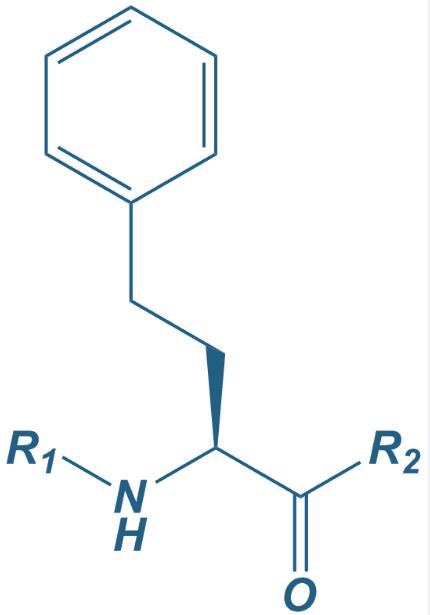
|
L-Methionine sulfone
|
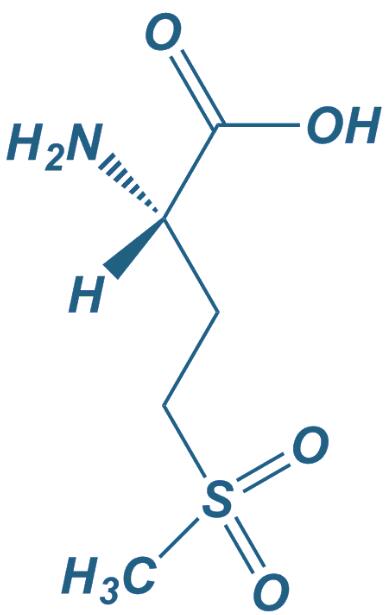
|
L-Methionine sulfoxide
|
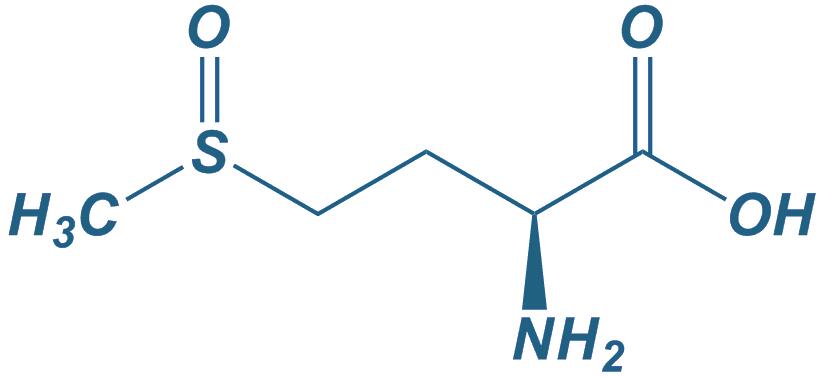
|
Lys(Ac)
|
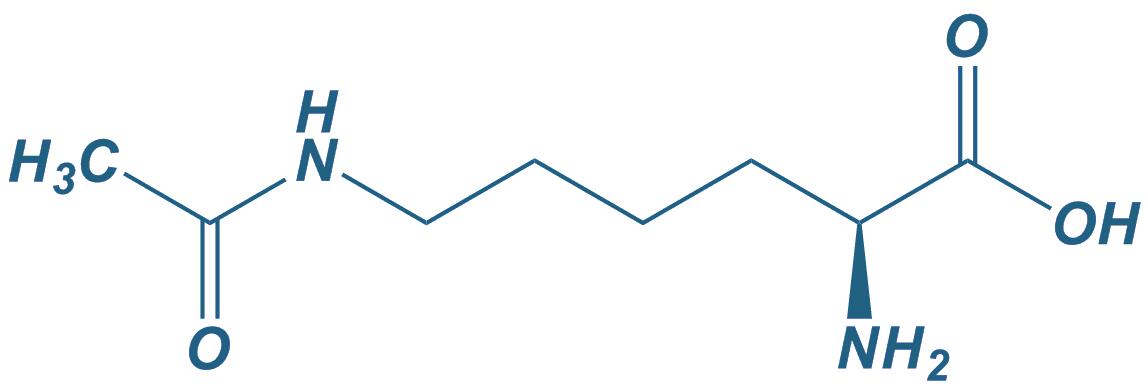
|
Lys(Fmoc)-OH
|
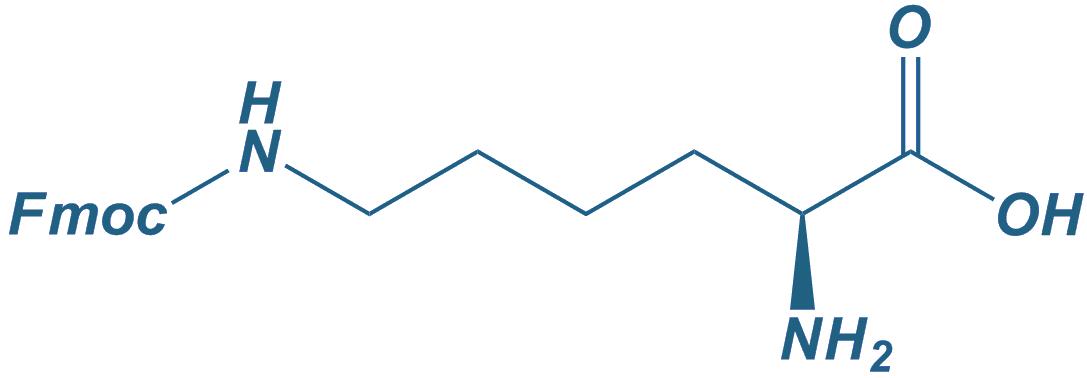
|
Naphtylalanine
|
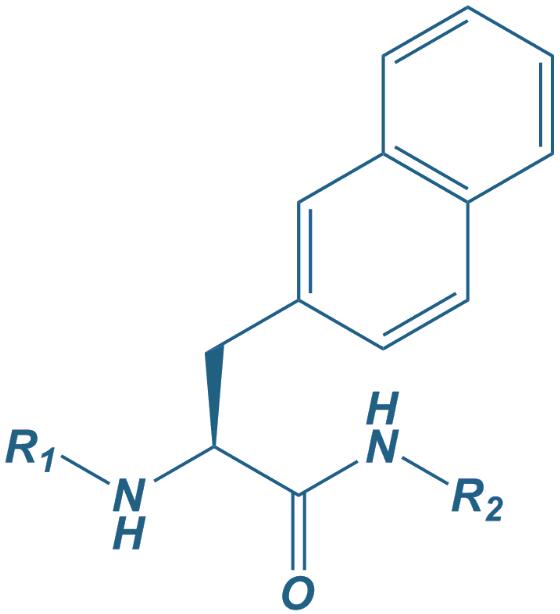
|
N-Methyl Phe
|
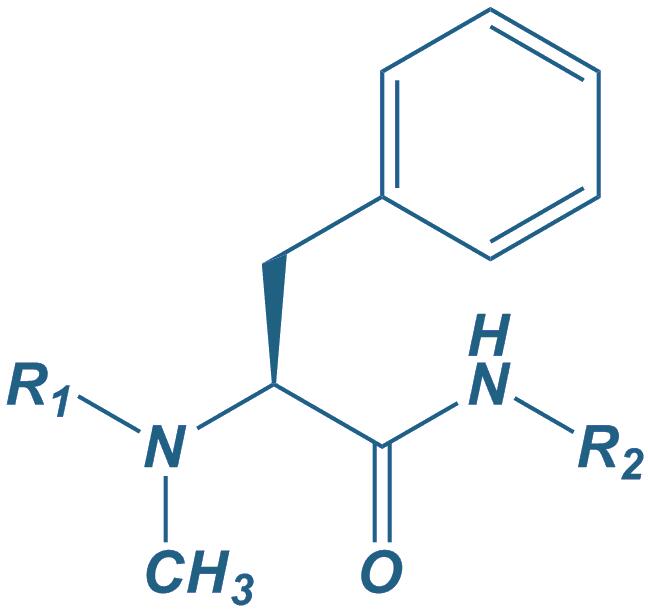
|
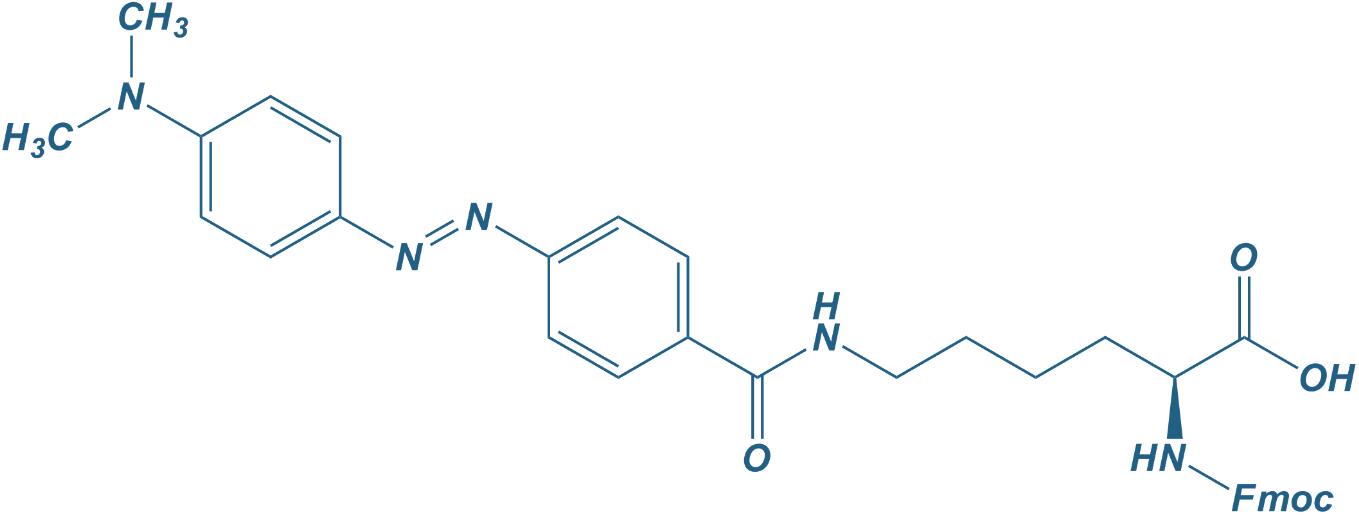
Nα-Fmoc-Nε-Dabcyl-L-Lys
|
|
|
