Fluorescence Resonance Energy Transfer is a method that allows the detection of a ‘distance-dependent’ interaction between the excited states of two distinct, dye-linked, molecules, i.e. the ‘fluorophore’ and the ‘quencher’. Quenched fluorescent peptides (‘FRET peptides’) are widely used as suitable substrates in enzyme studies.
Their synthesis requires that the peptide is conjugated with both a fluorophore and a quencher dye. ‘Fluorescence/quencher’ pairs do require distinct overlap between the fluorescence emission spectrum of the fluorophore and the UV-absorbance spectrum of the quencher. So, when the fluorophore and quencher are conjugated to one and the same peptide, with a limited distance, the quencher efficiently blocks the emission of the fluorophore. However, when a peptide bond is cleaved, e.g. by enzymatic degradation, the distance between fluorophore and quencher is suddently increased significantly, and as a result of this the fluorophore is activated. The fluorescence signal can be detected continuously, allowing quantification of the enzyme activity.
Frequently used fluorophore/quencher combinations are:
|
Fluorophore
|
Quencher
|
Excitation Wavelenghs
|
Emission Wavelenghs
|
|
Abz
2-Aminobenzoyl
|
Dnp
2,4-dinitrophenyl
|
320 nm
|
420 nm
|
|
EDANS
5-[(2-Aminoethyl) amino] naphthalene-1-sulfonic acid
|
Dabcyl
4-([4′-dimethylamino)phenyl] azo)benzoyl
|
340 nm
|
490 nm
|
|
Mca
7-Methoxycoumarin-4-yl)acetyl
|
Dnp
2,4-dinitrophenyl
|
325 nm
|
392 nm
|
|
Trp
Tryptophan
|
Dnp
2,4-dinitrophenyl
|
280 nm
|
360 nm
|
|
FAM
Carboxyfluorescein
|
Dabcyl
4-([4′-dimethylamino)phenyl]azo)benzoyl
|
492 nm
|
517 nm
|
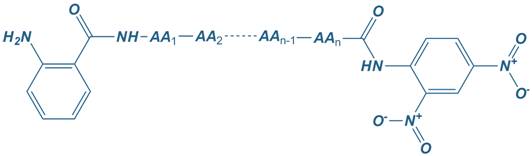
Abz – Dnp
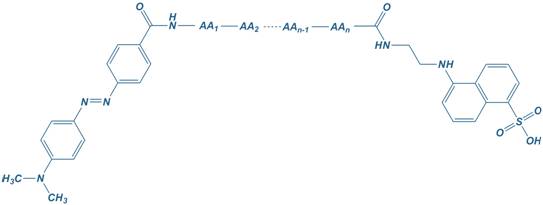
EDANS – Dabcyl
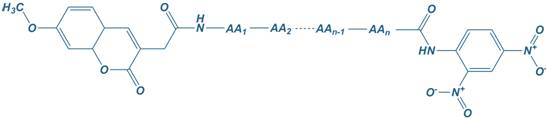
Mca – Dnp
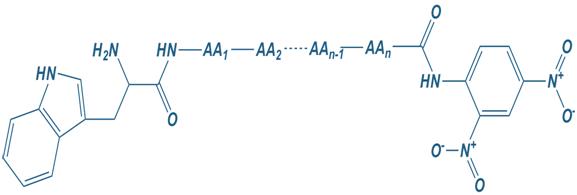
Tryptophan – Dnp
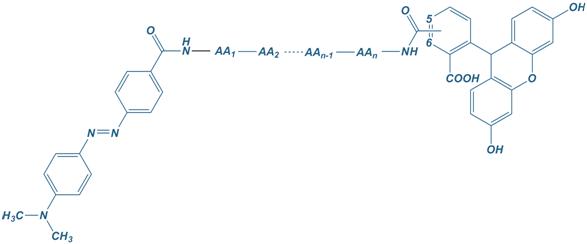
FAM – Dabcyl
|
