Protein prenylation involves the addition of a farnesyl (C15) or geranylgeranyl (C20) isoprenoid moiety onto a cysteine residue located near the carboxyl terminal of a protein. This posttranslational modification occurs at the C-terminus of approximately 2% of all mammalian proteins. Different classes of proteins, among them the family of Ras proteins, are known to be farnysylated. Prenylation is an important process to mediate protein-protein interactions and protein-membrane interactions. A recently discovered application of prenylated peptides is that they have inherent cell-penetrating ability.
Prenylation of peptides requires the addition of a lipid chain formed by three (farnesyl) or 4 (geranylgeranyl) isoprene units to a free thiol group. The synthesis of peptides containing farnesyl or geranylgeranyl groups is severely hampered by the pronounced acid-lability of these groups, in particular of the double bonds which are all transconfigured in the farnesyl and geranylgeranyl chains. Pepmic has developed a reliable procedure for the synthesis of prenylated peptides, and therefore is able supply these peptides at the request of its customers.
|
Name
|
Structure
|
Farnesyl
|
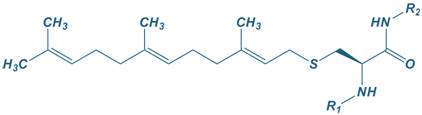
|
Geranyl
|
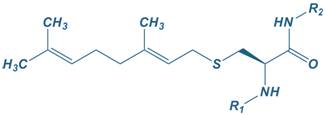
|
|
